physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
4.5 (221) In stock

The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In
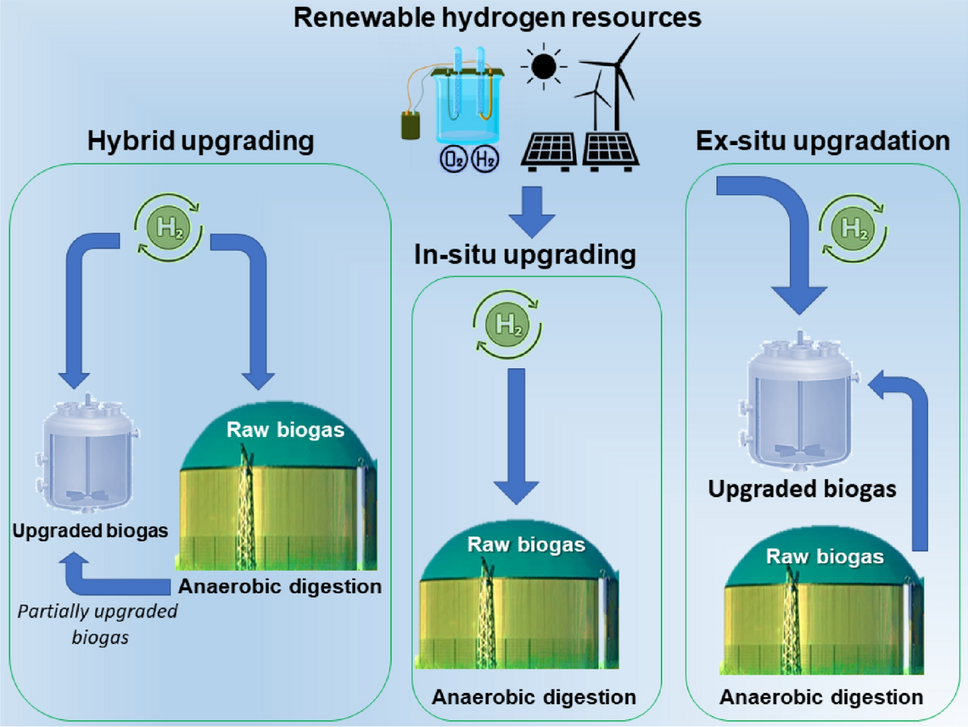
Integration of biogas systems into a carbon zero and hydrogen
Ideal gas - Wikipedia

Compressibility of Liquids - an overview

Metal–organic framework - Wikipedia

Non-Ideal Gas Behavior Chemistry: Atoms First

physical chemistry - why is the pressure exerted by ideal gas on

gas laws - Compressible Factor - Chemistry Stack Exchange

Description of real gases: Compression factor

Compressibility factor (gases) - Citizendium
Math cad compressibility factor, z, of real gas using the redlich-kwong equation of state
thermodynamics - Variation of compressiblity factor with temperature - Chemistry Stack Exchange
Compressibility factor z versus 100/V, for several values of
 E9 Olivia Climbing Pants Women - Ash
E9 Olivia Climbing Pants Women - Ash Scrunch Bum Leggings Wholesale - China Fitness Clothing
Scrunch Bum Leggings Wholesale - China Fitness Clothing Bollywood Brides: 10 Bridal Fashion Trends We Learned from Our Favorite Bollywood Celebs, Bridal Look
Bollywood Brides: 10 Bridal Fashion Trends We Learned from Our Favorite Bollywood Celebs, Bridal Look Corset Excelsior by Impremerie Moullot Original 1900 Vintage French Clothing Advertisement Stone Lithograph Antique Poster Linen
Corset Excelsior by Impremerie Moullot Original 1900 Vintage French Clothing Advertisement Stone Lithograph Antique Poster Linen Damen Sport-BH Calvin Klein Cotton Bralette Schwarz
Damen Sport-BH Calvin Klein Cotton Bralette Schwarz Swimsuits For All Women's Plus Size Tie Front Cup Sized Cap Sleeve Underwire Bikini Top 22 D/Dd Black
Swimsuits For All Women's Plus Size Tie Front Cup Sized Cap Sleeve Underwire Bikini Top 22 D/Dd Black